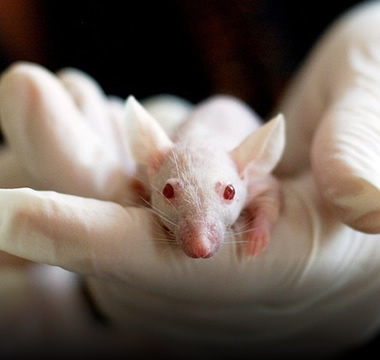
Preclinical Contract CRO
Service Portfolio
In Vivo Pharmacology
- In-Vivo efficacy studies using appropriate animal disease models
- In-vivo biomarker assays such as ELISA, Histology, Immunohistochemistry, RT-PCR, WB etc..
- PK-PD Investigation
- Invasive and Non-invasive pharmacological read outs.
- Validation of various surgical models
- New and rare disease model development
- Dose, route and regime optimization studies
Cell Biology
- In vitro cell based efficacy screening using validated models in multiple therapeutic areas
- Comprehensive mechanistic elucidation for various class of test items
- In vitro cytotoxicity assays for organ toxicity
- Stem cells based in vitro studies
- Employment of well-established hallmark end point technologies such as Flow cytometry, ELISA, western blotting, PCR, Microscopy etc.
- Efficacy testing of products for claim substantiation
- Wide-ranging panels of Biomarkers in multiple disease and wellness areas
Toxicology
- Acute & Sub-acute toxicity studies
- Sub-chronic & Chronic toxicity studies
- Developmental & Reproductive Toxicity (DART) Studies
- Neurotoxicity studies
DMPK
- In vitro ADME studies
- Pharmacokinetic studies
- Tissue Distribution studies
- PK-PD studies
- Toxicokinetic studies
- Biomarker studies
- Bioanalysis
Formulation Development
- Formulation Development
- Lab/Pilot to Commercial Scale Process Development
- Lab/Pilot batch Manufacturing for study
- Product Characterization
- Shelf-Life Evaluation – Stability Study
- Product Docket Preparation and Technology Transfer
- Support to Commercial Scaleup and Process Validation
Analytical Chemistry
- Herbal Product Characterization
- Dose formulation analysis for preclinical studies
- 5 Batch analysis
- Phys-chem studies
- Residue analysis
Genetic Toxicology
- AMES Test (Salmonella typhimurium & E.coli)
- In vitro & In vivo Chromosomal Aberration Test
- In vitro & In vivo Micronucleus Test
- In vitro Cell Gene Mutation Test
Service Portfolio
Why choose Althea
We offer smart, innovative solutions allowing our customers to solve urgent needs based on sustainability, efficiency and cost-effectiveness. Our services are tailored to fit specific needs and optimize performances while using less resources. We work towards brining speed to the programs of our clients.
All studies conducted at our facilities in India are in accordance with the regulatory requirements of the EMA, US FDA, OECD, AAALAC. Our professionalism and exceptional customer oriented skills have given us a competitive edge and we have become the chosen outsourcing partner for a diverse set of customers.
Science and Technology
Our commitment to scientific research allows us to adapt to the latest technological shifts...
Partnership Driven
Althea’s unique position of being a partner driven company gives us the ability to harness...
Diversified Portfolio & Interests
Althea’s diversified interests make us an integrated healthcare and wellness company...
Innovative Healthcare Products
Althea has always worked towards adding innovation to definition of our name itself i.e. “HEALING”...
sq ft
Dedicated Lab Space
sq ft
State Of The Art Vivarium
Instruments
Survelliance Monitoring
sq ft
Archival
Drug Development
Services
Our established scientific experience and customized, collaborative approach makes Althea the ideal partner for your company to achieve meaningful, high-quality, and fast results.
Together with Dabur Research Foundation, we provide scientific advice in selected therapeutic areas and a range of services easily adapted to your needs. With extensive experience in In Vitro, Ex-Vivo & In Vivo Pharmacology, Exploratory & GLP Toxicology & DMPK.
Visit our strategic partner website to know more : www.daburreseach.in
Our News & Events
- News
Our Partners
Our strong network of partners along with their specialized interests give us a unique positioning in the market, all leveraged through our own research and development infrastructure. We believe in creating an ecosystem of strategic alliances with our partners to help maximize their competitive advantage on mutually agreed terms toward a profitable business for all.
Accelerate your Preclinical Development
Althea along with its strategic partner Dabur Research Foundation guides and operates the technical and scientific needs of global biotech companies and pharmaceutical developers during the preclinical phase of research. We provide a solid foundation of laboratory & drug development services, disease models and expertise.
Start Project Services Overview